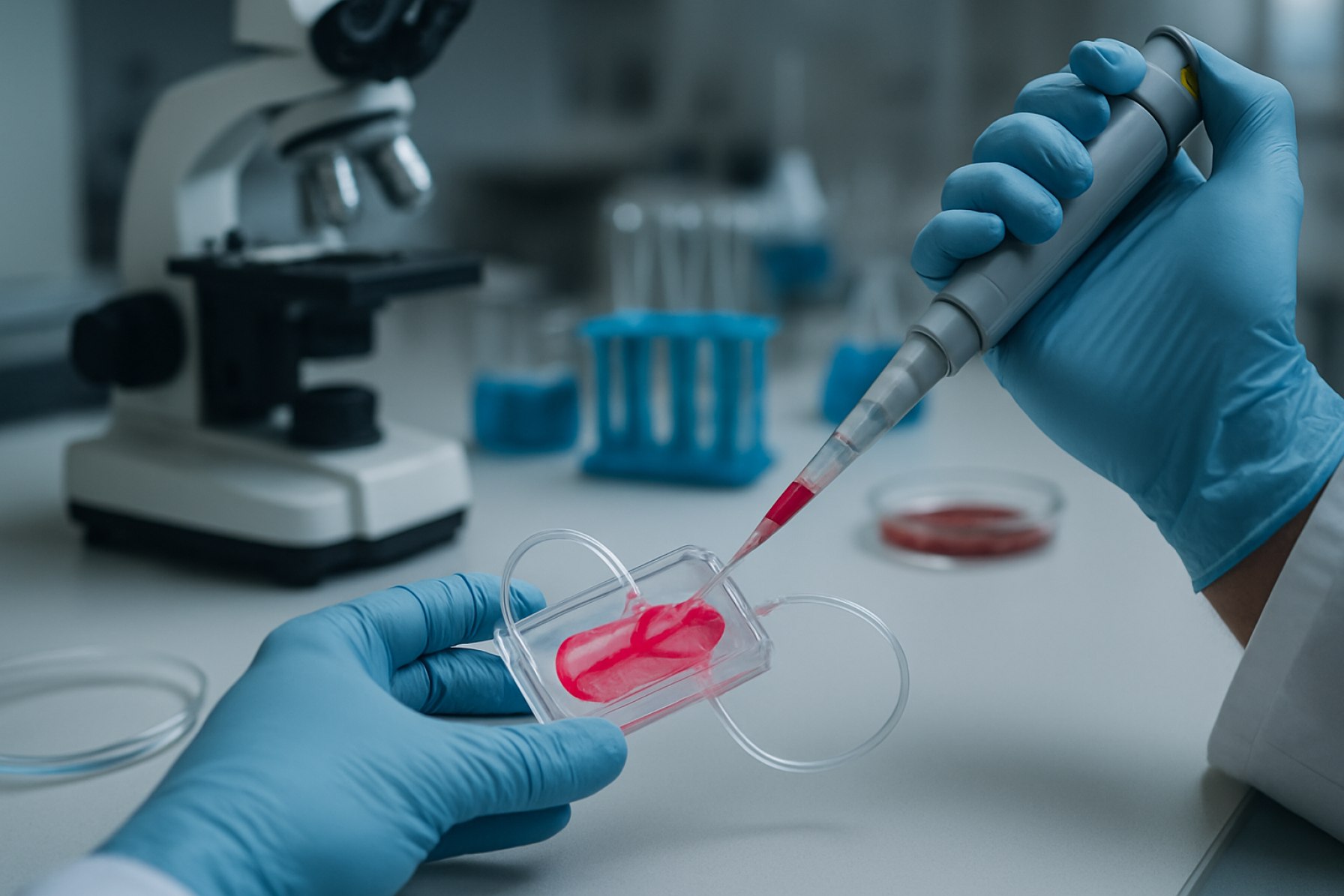
Biofabrication of Organ-on-a-Chip Systems in 2025: Transforming Drug Discovery and Personalized Medicine. Explore the Breakthroughs, Market Surge, and What the Next 5 Years Hold.
- Executive Summary: Key Insights and 2025 Highlights
- Market Overview: Defining Biofabrication of Organ-on-a-Chip Systems
- Market Size & Forecast (2025–2030): Growth Drivers, Trends, and CAGR Analysis (Estimated CAGR: 22% 2025–2030)
- Technology Landscape: Advances in Biofabrication, Microfluidics, and Biomaterials
- Competitive Analysis: Leading Players, Startups, and Strategic Partnerships
- Applications: Drug Discovery, Toxicology, Disease Modeling, and Personalized Medicine
- Regulatory Environment and Standardization Efforts
- Challenges and Barriers: Technical, Commercial, and Ethical Considerations
- Investment & Funding Trends: Venture Capital, Grants, and M&A Activity
- Future Outlook: Emerging Opportunities, Unmet Needs, and Market Projections to 2030
- Sources & References
Executive Summary: Key Insights and 2025 Highlights
The biofabrication of organ-on-a-chip systems is rapidly transforming biomedical research and preclinical drug testing by enabling the creation of microengineered platforms that closely mimic human organ physiology. In 2025, the field is marked by significant advancements in microfluidic design, biomaterial innovation, and integration of real-time sensing technologies. These developments are driving increased adoption across pharmaceutical, biotechnology, and academic sectors, as organ-on-a-chip systems offer more predictive and ethical alternatives to traditional animal models.
Key insights for 2025 highlight the convergence of 3D bioprinting and microfluidics, allowing for the precise spatial arrangement of multiple cell types and extracellular matrices within chip platforms. This has led to the emergence of multi-organ chips, or “body-on-a-chip” systems, which facilitate the study of complex inter-organ interactions and systemic drug responses. Companies such as Emulate, Inc. and MIMETAS B.V. are at the forefront, commercializing platforms that support high-throughput screening and disease modeling with unprecedented physiological relevance.
A notable trend in 2025 is the integration of advanced biosensors and AI-driven analytics, enabling continuous monitoring of cellular responses and more robust data interpretation. This is accelerating the validation of organ-on-a-chip models for regulatory acceptance, with organizations like the U.S. Food and Drug Administration (FDA) actively engaging in collaborative research to establish standardized protocols and performance benchmarks.
Sustainability and scalability are also in focus, with manufacturers investing in automated biofabrication processes and reusable chip materials to reduce costs and environmental impact. The expansion of open-source design repositories and collaborative consortia, such as those supported by the National Institutes of Health (NIH), is fostering innovation and accelerating the translation of organ-on-a-chip technologies from the lab to industry.
In summary, 2025 is poised to be a pivotal year for the biofabrication of organ-on-a-chip systems, characterized by technological maturation, broader industry adoption, and increasing regulatory engagement. These trends are expected to further solidify organ-on-a-chip platforms as essential tools for drug discovery, toxicology, and personalized medicine.
Market Overview: Defining Biofabrication of Organ-on-a-Chip Systems
The biofabrication of organ-on-a-chip systems represents a transformative convergence of tissue engineering, microfluidics, and biomaterials science. These systems are microengineered devices that mimic the physiological functions of human organs, providing a dynamic and controllable environment for studying organ-level responses. Unlike traditional cell culture or animal models, organ-on-a-chip platforms enable more accurate simulation of human biology, which is critical for drug development, disease modeling, and toxicity testing.
The market for biofabricated organ-on-a-chip systems is rapidly expanding, driven by the increasing demand for predictive, human-relevant models in pharmaceutical research and personalized medicine. Key players in the field, such as Emulate, Inc. and MIMETAS B.V., are advancing the development and commercialization of these platforms. Their technologies integrate living cells with microfluidic channels, enabling the recreation of tissue-tissue interfaces, mechanical forces, and biochemical gradients found in vivo.
Biofabrication techniques, including 3D bioprinting and micro-patterning, are central to the evolution of organ-on-a-chip systems. These methods allow for precise spatial arrangement of multiple cell types and extracellular matrix components, closely replicating the architecture and function of native tissues. For example, TissUse GmbH has developed multi-organ chips that connect different tissue types, facilitating the study of systemic interactions and pharmacokinetics.
Regulatory agencies and industry consortia, such as the U.S. Food and Drug Administration (FDA) and the European Federation of Pharmaceutical Industries and Associations (EFPIA), are increasingly recognizing the potential of organ-on-a-chip technologies to reduce reliance on animal testing and improve the efficiency of drug discovery pipelines. This recognition is fostering collaborations and funding initiatives aimed at standardizing and validating these systems for broader adoption.
As the field matures, the biofabrication of organ-on-a-chip systems is poised to play a pivotal role in the future of biomedical research, offering scalable, reproducible, and physiologically relevant models that bridge the gap between in vitro studies and clinical outcomes.
Market Size & Forecast (2025–2030): Growth Drivers, Trends, and CAGR Analysis (Estimated CAGR: 22% 2025–2030)
The global market for biofabrication of organ-on-a-chip systems is poised for robust expansion between 2025 and 2030, with an estimated compound annual growth rate (CAGR) of 22%. This growth is driven by increasing demand for physiologically relevant in vitro models in drug discovery, toxicology testing, and personalized medicine. The market size is projected to reach several billion USD by 2030, reflecting the rapid adoption of advanced biofabrication techniques and the integration of microfluidics, 3D bioprinting, and stem cell technologies.
Key growth drivers include the pharmaceutical industry’s need to reduce drug development costs and timelines, as organ-on-a-chip systems offer more predictive human-relevant data compared to traditional animal models. Regulatory agencies such as the U.S. Food and Drug Administration are increasingly supporting alternative testing methods, further accelerating market adoption. Additionally, the rise of precision medicine and the demand for patient-specific disease models are fueling investments in biofabrication platforms capable of replicating complex tissue architectures and functions.
Technological advancements are shaping market trends, with companies like Emulate, Inc. and MIMETAS B.V. pioneering scalable organ-on-a-chip solutions. The integration of artificial intelligence and automation in biofabrication workflows is enhancing throughput and reproducibility, making these systems more accessible for high-content screening applications. Furthermore, collaborations between academic institutions, industry players, and regulatory bodies are fostering innovation and standardization across the sector.
Geographically, North America and Europe are expected to maintain market leadership due to strong research infrastructure and supportive regulatory frameworks. However, Asia-Pacific is anticipated to witness the fastest growth, driven by expanding biotechnology sectors and increased government funding for life sciences research.
In summary, the biofabrication of organ-on-a-chip systems market is set for significant growth from 2025 to 2030, propelled by technological innovation, regulatory support, and the urgent need for more predictive and ethical preclinical testing models. The estimated 22% CAGR underscores the sector’s dynamic evolution and its critical role in shaping the future of biomedical research and drug development.
Technology Landscape: Advances in Biofabrication, Microfluidics, and Biomaterials
The technology landscape for the biofabrication of organ-on-a-chip (OoC) systems in 2025 is marked by rapid advances in biofabrication techniques, microfluidic engineering, and the development of novel biomaterials. These innovations are converging to create more physiologically relevant and scalable OoC platforms, which are increasingly used for drug discovery, disease modeling, and personalized medicine.
Biofabrication methods such as 3D bioprinting and photolithography have enabled the precise spatial arrangement of multiple cell types and extracellular matrix components within microfluidic devices. This level of control is essential for replicating the complex architecture and function of human tissues. For example, TissUse GmbH has developed multi-organ chips that integrate different tissue types, allowing for the study of inter-organ interactions under dynamic flow conditions.
Microfluidic technology remains at the core of OoC systems, providing the ability to mimic the mechanical and biochemical microenvironments of living organs. Advances in microfabrication, such as soft lithography and injection molding, have improved the reproducibility and scalability of chip production. Companies like Emulate, Inc. have commercialized microfluidic platforms that support the co-culture of human cells under controlled flow, shear stress, and chemical gradients, closely simulating in vivo conditions.
The choice and engineering of biomaterials are critical for the success of OoC devices. Recent progress in hydrogel chemistry and surface modification has led to the development of biomimetic substrates that support cell adhesion, differentiation, and function. For instance, MIMETAS B.V. utilizes proprietary gel-based matrices in their OrganoPlate® platform, enabling the formation of perfusable 3D tissue structures without artificial membranes.
Integration of sensors and real-time monitoring technologies is another significant trend. Embedded biosensors allow for continuous assessment of physiological parameters such as pH, oxygen, and metabolic activity, enhancing the utility of OoC systems for high-content screening and toxicity testing. Collaborative efforts between academic institutions and industry, such as those led by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), are accelerating the standardization and adoption of these advanced platforms.
Overall, the synergy between biofabrication, microfluidics, and biomaterials is driving the evolution of organ-on-a-chip systems toward greater complexity, reliability, and translational relevance in biomedical research.
Competitive Analysis: Leading Players, Startups, and Strategic Partnerships
The biofabrication of organ-on-a-chip (OoC) systems has rapidly evolved into a dynamic sector, driven by the convergence of microengineering, biomaterials, and cell biology. The competitive landscape is shaped by established biotechnology firms, innovative startups, and a growing network of strategic partnerships that accelerate research and commercialization.
Among the leading players, Emulate, Inc. stands out for its robust portfolio of organ-on-a-chip platforms, including liver, lung, and intestine chips, which are widely adopted in pharmaceutical R&D. MIMETAS is another key competitor, recognized for its OrganoPlate® technology that enables high-throughput screening and complex tissue modeling. CN Bio Innovations has also established a strong presence, particularly in liver-on-a-chip systems for drug metabolism and toxicity studies.
Startups are injecting fresh innovation into the field. Tissium and Nortis are notable for their focus on vascularized tissue models and microfluidic platforms, respectively. These companies leverage advanced biofabrication techniques, such as 3D bioprinting and microfluidic patterning, to create more physiologically relevant models. Tissium in particular is exploring the integration of bioactive materials to enhance tissue function and repair.
Strategic partnerships are a hallmark of the sector’s growth. Collaborations between technology providers and pharmaceutical companies, such as the partnership between Emulate, Inc. and F. Hoffmann-La Roche Ltd, aim to validate OoC platforms for preclinical drug testing. Academic-industry alliances, like those fostered by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), support the translation of academic breakthroughs into commercial products. Additionally, consortia such as the European Organ-on-Chip Society (EUROoCS) facilitate standardization and knowledge exchange across the sector.
Overall, the competitive environment in biofabricated organ-on-a-chip systems is characterized by rapid innovation, cross-sector collaboration, and a focus on scalability and regulatory acceptance. As the field matures, the interplay between established leaders, agile startups, and strategic partnerships will continue to shape its trajectory in 2025 and beyond.
Applications: Drug Discovery, Toxicology, Disease Modeling, and Personalized Medicine
The biofabrication of organ-on-a-chip (OoC) systems is revolutionizing several key areas in biomedical research and healthcare, notably drug discovery, toxicology, disease modeling, and personalized medicine. These microengineered devices, which replicate the microarchitecture and physiological functions of human organs, offer unprecedented opportunities to study human biology in vitro with high fidelity.
In drug discovery, OoC platforms enable more predictive preclinical testing by providing human-relevant data on drug efficacy and pharmacokinetics. Unlike traditional cell cultures or animal models, these systems can mimic organ-specific responses, reducing the risk of late-stage drug failures. For example, liver-on-a-chip devices are used to assess drug metabolism and hepatotoxicity, while heart-on-a-chip models evaluate cardiotoxic effects, streamlining the drug development pipeline for pharmaceutical companies such as Pfizer Inc. and Novartis AG.
Toxicology testing is another critical application, where OoC systems provide a more accurate assessment of chemical safety. Regulatory agencies like the U.S. Food and Drug Administration (FDA) are increasingly interested in these technologies as alternatives to animal testing, aligning with the global push for more ethical and human-relevant safety evaluations.
Disease modeling benefits significantly from the biofabrication of OoC systems. By incorporating patient-derived cells, researchers can recreate disease-specific microenvironments, enabling the study of complex pathologies such as cancer, neurodegenerative disorders, and rare genetic diseases. This approach is supported by organizations like the National Institutes of Health (NIH), which funds research into OoC-based disease models to better understand disease mechanisms and identify novel therapeutic targets.
Personalized medicine is perhaps the most transformative application. OoC devices fabricated with cells from individual patients allow for the testing of tailored treatment regimens, predicting patient-specific drug responses and minimizing adverse effects. Companies such as Emulate, Inc. are at the forefront of developing personalized OoC platforms, collaborating with healthcare providers to integrate these systems into clinical decision-making.
As biofabrication techniques advance, the integration of organ-on-a-chip systems into these applications is expected to accelerate, driving innovation in biomedical research and paving the way for safer, more effective therapies.
Regulatory Environment and Standardization Efforts
The regulatory environment for the biofabrication of organ-on-a-chip (OoC) systems is rapidly evolving as these technologies gain traction in drug development, toxicology, and disease modeling. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have recognized the potential of OoC platforms to improve preclinical testing by providing more physiologically relevant data compared to traditional cell cultures or animal models. In 2023, the FDA launched the Alternative Methods Working Group to explore the integration of advanced in vitro models, including OoC, into regulatory science, signaling a shift toward acceptance of these systems in safety and efficacy assessments.
Standardization is a critical focus area, as the lack of harmonized protocols and quality benchmarks can hinder the widespread adoption of OoC technologies. Organizations such as the ASTM International and the International Organization for Standardization (ISO) have initiated working groups to develop consensus standards for the design, fabrication, and validation of OoC devices. These efforts aim to ensure reproducibility, interoperability, and data comparability across different platforms and laboratories. For example, ASTM’s E55 Committee on Manufacture of Pharmaceutical and Biopharmaceutical Products is actively developing guidelines for the qualification of microphysiological systems, which include OoC devices.
In parallel, public-private partnerships and consortia, such as the National Center for Advancing Translational Sciences (NCATS) Tissue Chip Program, are collaborating with regulatory bodies to establish performance criteria and reference materials. These initiatives facilitate the creation of standardized validation datasets, which are essential for regulatory submissions and eventual market approval. Furthermore, the EMA Innovation Task Force provides scientific advice to developers of novel OoC technologies, helping to align product development with regulatory expectations.
Looking ahead to 2025, the regulatory landscape is expected to become more defined, with clearer pathways for the qualification and acceptance of biofabricated OoC systems in both pharmaceutical and clinical applications. Continued collaboration between industry, academia, and regulatory agencies will be crucial to address outstanding challenges related to standardization, validation, and data integrity, ultimately accelerating the safe and effective integration of OoC technologies into biomedical research and healthcare.
Challenges and Barriers: Technical, Commercial, and Ethical Considerations
The biofabrication of organ-on-a-chip (OoC) systems presents a transformative approach to modeling human physiology and disease, yet it faces significant challenges across technical, commercial, and ethical domains. Technically, the integration of multiple cell types, precise microfluidic control, and the recreation of complex tissue interfaces remain formidable hurdles. Achieving reproducibility and scalability in the fabrication process is particularly difficult, as even minor variations in cell sourcing or microenvironmental conditions can lead to inconsistent results. Furthermore, the long-term viability and functionality of tissues within chips are often limited by issues such as nutrient diffusion, waste removal, and mechanical stability. These technical barriers necessitate ongoing innovation in biomaterials, microengineering, and cell culture techniques, as highlighted by organizations like the National Institute of Biomedical Imaging and Bioengineering.
Commercialization of OoC technologies is impeded by high development costs, regulatory uncertainty, and the need for standardization. The transition from academic prototypes to robust, user-friendly products suitable for pharmaceutical or clinical settings requires significant investment in manufacturing infrastructure and quality control. Regulatory agencies, such as the U.S. Food and Drug Administration, are still developing frameworks for the validation and approval of OoC systems, which can slow market adoption. Additionally, the lack of universally accepted standards for performance and interoperability complicates the integration of OoC platforms into existing drug development pipelines.
Ethical considerations also play a critical role in the advancement of biofabricated OoC systems. While these technologies have the potential to reduce reliance on animal testing, concerns persist regarding the sourcing of human cells, especially when using primary tissues or stem cells. Issues of donor consent, privacy, and the potential for unintended use of biological materials must be addressed through transparent policies and oversight. Furthermore, as OoC systems become more sophisticated, questions arise about the moral status of engineered tissues, particularly in models that approach higher-order organ complexity or neural function. Ethical guidelines from bodies such as the National Academies of Sciences, Engineering, and Medicine are essential to navigate these emerging dilemmas.
In summary, the path toward widespread adoption of biofabricated organ-on-a-chip systems in 2025 is shaped by ongoing technical innovation, the establishment of commercial and regulatory pathways, and the careful consideration of ethical implications.
Investment & Funding Trends: Venture Capital, Grants, and M&A Activity
The biofabrication of organ-on-a-chip (OoC) systems has emerged as a dynamic sector within the life sciences, attracting significant investment and funding activity in recent years. As of 2025, venture capital (VC) interest in OoC startups continues to grow, driven by the technology’s potential to revolutionize drug discovery, toxicity testing, and personalized medicine. Leading VC firms are increasingly backing companies that integrate advanced biofabrication techniques—such as 3D bioprinting and microfluidics—to create more physiologically relevant models of human organs. Notable examples include investments in startups like Emulate, Inc. and MIMETAS, which have secured multi-million dollar funding rounds to expand their platforms and commercial reach (Emulate, Inc., MIMETAS).
In addition to private investment, public and governmental grants play a crucial role in supporting early-stage research and development. Agencies such as the National Institutes of Health and the European Commission have launched dedicated funding calls for organ-on-a-chip and biofabrication projects, recognizing their potential to reduce animal testing and accelerate biomedical innovation. These grants often target collaborative projects between academia and industry, fostering the translation of novel biofabrication methods into scalable OoC products.
Mergers and acquisitions (M&A) activity in the OoC space has also intensified, as established life science and pharmaceutical companies seek to integrate organ-on-a-chip capabilities into their R&D pipelines. Recent years have seen strategic acquisitions of innovative OoC startups by major players such as CN Bio Innovations and InSphero AG, aiming to broaden their technology portfolios and accelerate product development. These M&A moves are often motivated by the desire to access proprietary biofabrication technologies, specialized expertise, and established customer networks.
Overall, the investment landscape for biofabricated organ-on-a-chip systems in 2025 is characterized by robust VC funding, substantial public grants, and increasing M&A activity. This influx of capital and strategic interest is expected to drive further innovation, scale-up, and commercialization, positioning OoC technologies as a cornerstone of next-generation biomedical research and drug development.
Future Outlook: Emerging Opportunities, Unmet Needs, and Market Projections to 2030
The future of biofabrication in organ-on-a-chip (OoC) systems is poised for significant advancement, driven by technological innovation, expanding applications, and increasing demand for physiologically relevant models in drug discovery and personalized medicine. As we approach 2030, several emerging opportunities and unmet needs are shaping the trajectory of this field.
One of the most promising opportunities lies in the integration of advanced biofabrication techniques, such as 3D bioprinting and microfluidics, to create more complex and functional OoC platforms. These innovations enable the precise spatial arrangement of multiple cell types and extracellular matrices, closely mimicking native tissue architecture and function. This progress is expected to enhance the predictive power of OoC systems in preclinical testing, reducing reliance on animal models and improving translational outcomes for human health.
Unmet needs remain, particularly in the standardization and scalability of biofabricated OoC devices. Current challenges include reproducibility, long-term cell viability, and the integration of real-time sensing technologies. Addressing these issues is critical for widespread adoption in pharmaceutical and clinical settings. Industry collaborations and regulatory engagement, such as those led by the U.S. Food and Drug Administration and the European Medicines Agency, are expected to play a pivotal role in establishing guidelines and validation frameworks for these emerging technologies.
Market projections indicate robust growth for the biofabricated OoC sector through 2030. The increasing emphasis on personalized medicine, coupled with the need for more accurate disease models, is driving investment from both public and private sectors. Major industry players, including Emulate, Inc. and MIMETAS B.V., are expanding their portfolios to address a broader range of organ systems and disease states. Additionally, partnerships with academic institutions and pharmaceutical companies are accelerating the translation of research prototypes into commercially viable products.
Looking ahead, the convergence of biofabrication, artificial intelligence, and high-throughput screening is expected to unlock new frontiers in OoC technology. These advances will not only address current limitations but also open avenues for applications in regenerative medicine, toxicity testing, and precision therapeutics, positioning biofabricated organ-on-a-chip systems as a cornerstone of next-generation biomedical research and healthcare.
Sources & References
- Emulate, Inc.
- MIMETAS B.V.
- National Institutes of Health (NIH)
- TissUse GmbH
- European Federation of Pharmaceutical Industries and Associations (EFPIA)
- National Institute of Biomedical Imaging and Bioengineering (NIBIB)
- Tissium
- Tissium
- F. Hoffmann-La Roche Ltd
- European Organ-on-Chip Society (EUROoCS)
- Novartis AG
- European Medicines Agency (EMA)
- ASTM International
- International Organization for Standardization (ISO)
- National Center for Advancing Translational Sciences (NCATS) Tissue Chip Program
- National Academies of Sciences, Engineering, and Medicine
- Emulate, Inc.
- European Commission
- InSphero AG